We are a
strategic
venture fund
Our raison d'être
We invest in the innovators behind the miracles of science who are passionate about improving human health. Sanofi Ventures serves as an entry point to the innovation ecosystem for Sanofi, strategically investing in early-stage transformational science and technology, when companies are too early to partner with or acquire.
Our Approach
We invest in top-tier biotech and digital health companies that focus on helping patients and transforming the practice of medicine. We invest across all stages of the private company lifecycle, from Seed to Series B and beyond, while leading financings, serving on boards, and taking pride in working alongside portfolio companies to drive long-term value.
We love to meet entrepreneurs and hear about what they are doing. Feel free to reach out to us…
Jason joined Sanofi Ventures in 2014 with a background in early-stage biotech investing and transactions. Prior to Sanofi, Jason was the Director of Corporate Development at Translate Bio (fka RaNA Therapeutics), a company co-founded by his previous firm Atlas Venture. While at Atlas, Jason was an Associate in the Life Sciences group. Previously, he was a Flagship Ventures Entrepreneurial Fellow, and consulted for the technology transfer company at the University of Cambridge while performing his doctoral research. Before his time at Cambridge, Jason was an analyst at JSB Partners LP, an investment banking firm, focusing on advisory and business development activities in the life sciences space.
Jason’s active investments include Abcuro, AdvanCell, Atalanta Therapeutics, Character Bio, Expedition Therapeutics, Glycomine, Matchpoint Therapeutics, Nura Bio, ReCode Therapeutics, Star Therapeutics, and Vrata. Previous investments include Escient Pharmaceuticals (acquired by Incyte), Q32 Bio (NASDAQ: QTTB), Icosavax (acquired by AstraZeneca), Kymera Therapeutics (NASDAQ: KYMR), Ovid Therapeutics (NASDAQ: OVID), Science 37 (NASDAQ: SNCE), and Lysosomal Therapeutics Inc. (acquired by Bial). Jason also serves as the Chair of the Board of Trustees of the Buckingham Browne and Nichols school and the Board of Directors of The Magdalene College Foundation.
Jason graduated with honors from Bowdoin College, and holds a Ph.D. from the University of Cambridge.
Laia joined Sanofi Ventures in 2018 with a background in early-stage biotech investing and drug development. Prior to Sanofi, Laia served as Investment Director for Ysios Capital where she led investments and served on the board of multiple early-stage companies.
Earlier in her career, Laia was part of the European New Business Development team of Janssen-Cilag, a pharmaceutical company of the Johnson & Johnson group, where she assessed commercial and scientific licensing-in opportunities. Previously she worked as a researcher in the UK in companies such as Spirogen (now AstraZeneca), Medivir and UCB-Celltech.
Laia's active investments include Eligo Bioscience, Granite Bio, Muna Therapeutics, NodThera, OMass Therapeutics, QurAlis, SpliceBio, T-Therapeutics and Therini Bio. Previous investments and board roles include Lava Therapeutics (LVTX).
Laia graduated in Chemistry from the University of Barcelona, where she also completed a Master in Science and a Ph.D. with honors. Laia holds an M.B.A. from Cambridge Judge Business School, University of Cambridge, where she focused on biotechnology, healthcare and finance.
Cris joined Sanofi Ventures in 2020 as a Partner to lead investments in Digital Health and Artificial Intelligence. He brings 20+ years of experience in dealmaking, building startups, scaling emerging technologies, and creating innovation platforms for some of the world’s largest organizations.
Previously, Cris was a founding member of Johnson & Johnson Innovation, serving as Global Head of Digital Innovation where he held chief digital responsibilities across J&J’s 250 operating companies in Pharma, Consumer, and Medical Devices. Before that, he spent six years at Novartis, leading emerging technology programs within Research Informatics.
With entrepreneurial DNA, Cris is notably known for his role in the innovation economy since co-founding Ultra Light Startups, one of the largest and oldest startup venture pitch accelerators in the U.S. This platform catalyzed hundreds of companies in Boston, NYC, and Silicon Valley, leading to several acquisitions by major tech companies. Cris was named to the Boston Business Journal's 40 Under 40 list and recognized as a Top 40 Healthcare Transformer by MM&M Magazine.
Cris currently serves on the boards of Aetion, Carbon Health, Click Tx, Empatica, Medisafe, Nucleai, Omada, and QuantHealth. He holds a M.Sc. in Computer Information Sciences from Boston University and a B.S. in Business Administration from Suffolk University.
Paulina joined Sanofi Ventures in 2022 with a background in early stage biotech investing and company building. Paulina’s active investments include Attovia, Avilar, Latigo, Odyssey, NextPoint, Normunity, Nuvig, Tisento, Vrata and ZagBio. Prior investments include Aliada Therapeutics (acquired by Abbvie).
Prior to joining Sanofi, Paulina was a Principal on the investment team at Omega Funds. While there Paulina incubated and served as the founding investor for Scorpion Therapeutics (acquired by Eli Lilly). Paulina’s investments also included Arrakis, IFM, Ikena (NASDAQ: IKNA), Synthekine, Theseus (NASDAQ: THRX), and Vanqua Bio.
Paulina began her career with Polaris Partners. Paulina served on the boards of Kala Pharmaceuticals (NASDAQ: KALA), Neuronetics (NASDAQ: STIM), Microchips Biotech (acquired by Dare Biosciences), Arsenal Medical and CAMP4 Therapeutics (NASDAQ: CAMP), where she was the Founding CEO. Paulina completed her postdoctoral fellowship in Robert Langer’s lab in the Chemical Engineering department at MIT. During her time at MIT, Paulina founded the MIT Postdoctoral Association and served as its President. Paulina also served on the MIT Intellectual Property Committee. Paulina completed her PhD in Molecular Medicine from the Wake Forest University School of Medicine and graduated magna cum laude from East Carolina University with a quadruple major in biochemistry, neuroscience, biology and chemistry. Paulina attended East Carolina University on a full athletic scholarship and served as the captain of the women’s varsity tennis team.
Francesc joined Sanofi Ventures in 2024 with a background in early-stage biotech investing, technology transfer, and biotech management. Prior to his role at Sanofi, he spent three years at AdBio Partners, a pan-European venture firm specializing in early-stage therapeutic projects, where he was responsible for scouting and conducting due diligence on new investment opportunities. As one of the first hires in Barcelona, he played a key role in establishing the local office and expanding the fund's presence in the region.
Earlier in his career, Francesc served as an operational manager at Kiji Therapeutics, an emerging gene and cell therapy company, and as an innovation manager at the technology transfer office of the University of Barcelona.
Francesc holds a degree in Biochemistry from the University of Barcelona, where he also completed an MBA with a focus on biotech investments.
In addition to his professional roles, Francesc serves on the therapeutics board of YVC Collective, a network of emerging life sciences venture capital professionals.
Chris joined Sanofi Ventures in 2017 with a background in life science and healthcare consulting. He previously spent three years as a management consultant at L.E.K. consulting where his work focused on corporate strategy, due diligence, commercial launch planning and asset valuation across the pharmaceutical, biotech, R&D and API manufacturing sectors.
Chris currently serves on the Boards of AdvanCell Isotopes, i2O Therapeutics, Minervax, and Sudo Bio, and is a Board observer for Atalanta Therapeutics, Avilar Therapeutics, Matchpoint Therapeutics, and Nextpoint Therapeutics. He also led Sanofi Ventures’ investment in Curevo.
Prior to a postdoctoral fellowship at Harvard University, Chris earned a Ph.D. in Chemistry from the University of North Carolina at Chapel Hill as well as a B.S. in both Biology and Chemistry from Roger Williams University.
Pierre joined Sanofi Ventures in 2024 with a background in biopharma investing. He previously worked as Analyst at Jeito Capital, where his areas of focus included oncology, cardiology, and neuroscience. Prior to Jeito Capital, Pierre worked as Researcher at Smart Immune, clinical-stage startup company developing cell therapies, as well as at Institut Imagine, leading European Center for research on genetic diseases
Pierre holds a Life Science Engineer’s degree from AgroParisTech, a MSc and a PhD in Biotechnology and Biotherapies from Université Paris Cité, and an MBA from the Collège des Ingénieurs. Before starting his doctoral program, Pierre was trained at Pasteur Institute and was a visiting student at the Massachusetts Institute of Technology.
Meg joined Sanofi Ventures in 2021 with a background in biotech investing and business development. She previously worked as Director of Business Development at Vertex Pharmaceuticals, where she was part of the External Innovation team. Prior to Vertex, Meg worked at RA Capital, a crossover investment fund, where her areas of focus included rare diseases, genetic therapies, and neuroscience.
Meg currently serves on the Board of Directors for Veralox.
Meg holds a Ph.D. in Neuroscience from Massachusetts Institute of Technology and a B.S. with Honors in Neuroscience from The Pennsylvania State University. She currently sits on the Steering Committee of the New England Venture Network.
Steve joined his current role in Sanofi Ventures in 2025 with a broad background across the pharma value chain. Steve joined Sanofi in 2020 and has been the legal partner for a variety of strategic investments, licensing, collaboration, and other related complex transactions, and has also been a key member of Sanofi product teams advancing pipeline and commercial assets.
Prior to Sanofi, Steve was in-house at Fresenius Medical Care, providing overarching strategic advice to Fresenius’ pharma and specialty pharmacy divisions.
Steve began his legal career at Foley Hoag LLP, where he handled transaction and IP disputes for pharma and biotech companies.
Steve earned a JD from Northwestern School of Law, cum laude and Order of the Coif, and a BA in Philosophy, summa cum laude, from the University of Illinois at Chicago. Outside of work, Steve runs an artist residency, plays in a jazz ensemble, and enjoys hiking in Vermont.
Frédérique joined Sanofi Ventures in April 2025. She previously worked in various departments at Sanofi for nearly 25 years, including Global Marketing, Early Development in R&D, and Partnering BD&L Operations.
Frédérique holds a post-graduate certificate in Executive Management and has a Bachelor's degree in Office Management.
She likes sports, music, nature, cooking, cinema, and spending time at home with her 3 children and family.
Anne joined Sanofi Ventures in May 2023. She previously worked in various departments over the last 19 years including Digital Vaccines, business development and global digital. Anne holds a Bachelors of Science in Business Administration degree with concentration in Management. In her spare time, she enjoys the beach, walking, exploring new trails with her husband, twins and two dogs.

Diagonal Therapeutics is a company advancing novel disease-modifying clustering antibodies that repair dysregulated signaling implicated in a range of illnesses with a lead program (DIAG723) being advanced in hereditary hemorrhagic telangiectasia (HHT) and pulmonary arterial hypertension (PAH).

Trial Library has developed a suite of AI-powered products that streamline patient recruitment and patient navigation for clinical trials. By integrating directly into healthcare workflows and mining EHR data across a national network of 1,500+ providers in 320+ community clinics, the platform identifies eligible participants faster, improves diversity, and reduces enrollment delays. Its solution combines AI-driven pre‑screening, engagement, and retention with a care navigation layer that supports patients throughout the trial journey, enabling equitable access at scale and higher completion rates. Sponsors use Trial Library to accelerate recruitment, optimize trial execution, lower costs, and bring therapies to market faster.

Lifordi Immunotherapeutics is a clinical-stage biotechnology company that leverages the success of antibody-drug conjugates (ADCs) to treat autoimmune and inflammatory disorders while applying its novel delivery platform to a broad range of payloads, including small molecules, ASOs, and siRNA.


- Sanofi and QuantHealth team up to advance AI-powered digital twins and clinical trial
- simulations to accelerate drug development
Tel Aviv & Cambridge, MA – October 1st, 2025 — QuantHealth, a pioneer in AI-driven clinical trial simulation, announced a strategic investment from Sanofi Ventures, the venture capital arm of global healthcare leader Sanofi. The investment will accelerate QuantHealth’s efforts to bring scalable, patient-level simulations and digital twin technologies to the forefront of drug development, bringing its total funds raised to $30 million.
QuantHealth’s platform enables pharmaceutical companies to virtually simulate clinical trials using real-world data and advanced AI models. By generating millions of patient-level digital twins, the platform predicts trial outcomes and optimizes protocol design, aiming to improve trial success rates, timelines, and cost efficiency.
The investment has further catalyzed an enterprise strategic relationship that supports Sanofi’s focus on integrating advanced AI and digital technologies to drive transformation across its R&D ecosystem. As part of the investment, Cris De Luca, Partner at Sanofi Ventures, will join as an observer on QuantHealth’s Board of Directors.
“We’re proud to welcome Sanofi Ventures as an investor and strategic ally in our journey,” said Orr Inbar, CEO and Co-founder of QuantHealth. “Sanofi is at the forefront of digital transformation in pharma, and this relationship will help us scale our impact and bring more predictive, AI-driven approaches to clinical development.”
“QuantHealth has the potential to transform how clinical trials are designed and optimized,” said Cris De Luca, Partner at Sanofi Ventures. “Their approach to leveraging digital twins and real-world data is advancing the next generation of R&D, and I look forward to supporting the team.”
“At Sanofi, we are building an AI-first organization, and engaging with innovators like QuantHealth is central to our strategy,” said Emmanuel Frenehard, Chief Digital Officer at Sanofi. “Their platform represents an important opportunity to reimagine clinical trial design through simulation, and we’re excited to explore this capability together.”
This investment and enterprise relationship underscores a shared commitment to advancing drug development through the responsible use of artificial intelligence in life sciences.
About QuantHealth
QuantHealth is an AI-driven clinical trial simulation company that helps pharmaceutical companies
design faster, more successful trials using real-world data and predictive modeling. With access to over 350 million patient records and proprietary AI algorithms, QuantHealth’s platform simulates trials at scale to optimize protocols, reduce risk, and accelerate timelines.
About Sanofi Ventures
Sanofi Ventures is the corporate venture capital arm of Sanofi, investing globally in early-stage biotech and digital health companies aligned with Sanofi’s mission to chase the miracles of science. Areas of focus include immunology, oncology, rare diseases, vaccines, and digital innovation.



- Sanofi receives exclusive rights to commercialize losmapimod in all territories outside the U.S.; Fulcrum retains full U.S. commercialization rights
- Fulcrum will receive an upfront payment of $80.0 million, and is eligible to receive $975.0 million in potential milestones, plus royalties on ex-U.S. product sales; parties will share future global development costs 50:50
- Conference call and webcast scheduled for 8:00 a.m. ET today to discuss the collaboration and other recent corporate developments, in conjunction with the first quarter 2024 financial results
CAMBRIDGE, Mass., May 13, 2024 (GLOBE NEWSWIRE) -- Fulcrum Therapeutics, Inc.® (Fulcrum) (Nasdaq: FULC), a clinical-stage biopharmaceutical company focused on developing small molecules to improve the lives of patients with genetically defined rare diseases, today announced that it has entered into a collaboration and license agreement with Sanofi (Nasdaq: SNY) for the development and commercialization of losmapimod, an oral small molecule being investigated for the treatment of facioscapulohumeral muscular dystrophy (FSHD). Under the collaboration and license agreement, Sanofi obtains exclusive commercialization rights for losmapimod outside of the U.S.
The collaboration and license agreement combines Fulcrum’s expertise in FSHD with Sanofi’s global reach and unparalleled commitment to treating patients with rare diseases. Losmapimod is currently being evaluated in a global Phase 3 clinical trial for the treatment of FSHD, a chronic and progressive genetic muscular disorder that is characterized by significant muscle cell death and fat infiltration into muscle tissue. Results from ReDUX4, the Phase 2 clinical trial evaluating losmapimod for the treatment of FSHD, demonstrated a slowing of disease progression and improved muscle health. Fulcrum expects to report topline data from REACH, the global Phase 3 clinical trial, in the fourth quarter of 2024. Following positive data from the Phase 3 trial, Fulcrum and Sanofi plan to submit marketing applications in the U.S., Europe, Japan, and other geographies.
“Sanofi is a proven leader in developing therapeutics for rare neuromuscular diseases and is the ideal partner to maximize the opportunity and reach of losmapimod outside the U.S.,” said Alex C. Sapir, Fulcrum’s president and chief executive officer. “This deal aligns with our core strategy, allowing Fulcrum to remain focused on preparations for commercialization of losmapimod in the U.S., while leveraging Sanofi’s exceptional global commercial capabilities and established infrastructure in key markets around the world. We are excited about the potential to provide the first approved treatment for FSHD patients, and we look forward to working with Sanofi to bring losmapimod to patients globally.”
“This partnership provides an exciting opportunity to expand Sanofi’s rare disease franchise and deliver the first approved FSHD treatment to patients with the strength and reach of our commercial organization,” said Burcu Eryilmaz, Sanofi’s Global Head of Rare Diseases. “Losmapimod has shown meaningful clinical benefits that underscore the disease-modifying potential and opportunity to address the high unmet need for a safe and effective drug that slows disease progression. With a deep commitment to bringing hope and new treatment options to patients, we look forward to working closely with Fulcrum as losmapimod advances through late-stage development.”
Per the terms of the agreement, Fulcrum will receive an upfront payment of $80.0 million and is eligible to receive up to an additional $975.0 million in specified regulatory and sales-based milestones, along with tiered escalating royalties starting in the low-teens on annual net sales of losmapimod outside the U.S. In addition, Fulcrum and Sanofi will equally share future global development costs.
Conference Call and Webcast
Individuals may register for the conference call by clicking the link here. Once registered, participants will receive dial-in details and unique PIN which will allow them to access the call. An audio webcast will be accessible through the Investor Relations section of Fulcrum’s website at www.fulcrumtx.com or by clicking here. Following the live webcast, an archived replay will also be available.
About Losmapimod
Losmapimod is a selective p38α/β mitogen activated protein kinase (MAPK) inhibitor. Fulcrum exclusively in-licensed losmapimod from GSK following Fulcrum’s discovery of the role of p38α/β inhibitors in the reduction of DUX4 expression and an extensive review of known compounds. Results reported from the Phase 2 ReDUX4 trial demonstrated a slowing of disease progression and improved function, including positive impacts on upper extremity strength and functional measures supporting losmapimod’s potential to be a transformative therapy for the treatment of FSHD. Although losmapimod had never previously been explored in muscular dystrophies, it had been evaluated in more than 3,600 subjects in clinical trials across multiple other indications, with no safety signals attributed to losmapimod. Losmapimod has been granted U.S. Food and Drug Administration (FDA) Fast Track designation and Orphan Drug Designation for the treatment of FSHD. Losmapimod is currently being evaluated in a Phase 3 multi-center randomized, double-blind, placebo-controlled, 48-week parallel-group study in people with FSHD (NCT05397470).
About FSHD
FSHD is a serious, rare, progressive and debilitating disease for which there are no approved treatments. It is characterized by fat infiltration of skeletal muscle leading to muscular atrophy involving primarily the face, scapula and shoulders, upper arms, and abdomen. Impact on patients includes profound decreases in the ability to perform activities of daily living, loss of upper limb function, loss of mobility and independence and chronic pain. FSHD is one of the most common forms of muscular dystrophy and has an estimated patient population of 30,000 in the United States alone.
About Fulcrum Therapeutics
Fulcrum Therapeutics is a clinical-stage biopharmaceutical company focused on developing small molecules to improve the lives of patients with genetically defined rare diseases in areas of high unmet medical need. Fulcrum’s two lead programs in clinical development are losmapimod, a small molecule in development for the treatment of facioscapulohumeral muscular dystrophy (FSHD), and pociredir (formerly known as FTX-6058), a small molecule designed to increase expression of fetal hemoglobin and in development for the treatment of sickle cell disease (SCD). Fulcrum uses proprietary technology to identify drug targets that can modulate gene expression to treat the known root cause of gene mis-expression. For more information, visit www.fulcrumtx.com and follow us on Twitter/X (@FulcrumTx) and LinkedIn.
Forward-Looking Statements
This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this press release are forward-looking statements, including express or implied statements regarding Fulcrum’s collaboration and license agreement with Sanofi and receipt of the upfront payment thereunder; its ability to receive the milestone and royalty payments thereunder and achieve benefits therefrom; timing of data from REACH and its ability to support submission of marketing applications for losmapimod; and Fulcrum’s ability to deliver an FDA-approved therapy for FSHD patients; among others. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Any forward-looking statements are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in, or implied by, such forward-looking statements. These risks and uncertainties include, but are not limited to, risks associated with Fulcrum’s ability to continue to advance its product candidates in clinical trials; initiating and enrolling clinical trials on the timeline expected or at all; obtaining and maintaining necessary approvals from the FDA and other regulatory authorities; replicating in clinical trials positive results found in preclinical studies and/or earlier-stage clinical trials of losmapimod, pociredir and any other product candidates; obtaining, maintaining or protecting intellectual property rights related to its product candidates; managing expenses; managing executive and employee turnover, including integrating a new CMO; and raising the substantial additional capital needed to achieve its business objectives, among others. For a discussion of other risks and uncertainties, and other important factors, any of which could cause Fulcrum’s actual results to differ from those contained in the forward-looking statements, see the “Risk Factors” section, as well as discussions of potential risks, uncertainties, and other important factors, in Fulcrum’s most recent filings with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent Fulcrum’s views as of the date hereof and should not be relied upon as representing Fulcrum’s views as of any date subsequent to the date hereof. Fulcrum anticipates that subsequent events and developments will cause Fulcrum’s views to change. However, while Fulcrum may elect to update these forward-looking statements at some point in the future, Fulcrum specifically disclaims any obligation to do so.
Contact:
Chris Calabrese
LifeSci Advisors, LLC
ccalabrese@lifesciadvisors.com
917-680-5608


Collaboration will focus on co-development of unique datasets to understand health in everyday life
January 10, 2022 06:00 AM Eastern Standard Time
SAN MATEO, Calif.--(BUSINESS WIRE)--Evidation, the company creating new ways to measure and improve health in everyday life, is expanding its decade-long collaboration with Sanofi to build upon their joint real-world data initiatives. This new phase will focus on the co-development of unique datasets to develop and validate new measures of health and wellness.
Evidation’s collaboration with Sanofi has delivered groundbreaking results to date, with over 20 studies conducted across 10 therapeutic areas, including diabetes and Type 2 Inflammation, more than 500,000 patients reached, and four studies published. This continued collaboration will further the work Evidation and Sanofi have pioneered to translate person-generated health data into quantified clinical and economic outcomes, a key priority for both companies.
“After nearly a decade of working with Sanofi, we are proud to expand this collaboration agreement to advance the role that real-world data and analysis can play in better understanding health and disease,” said Christine Lemke, Evidation co-founder and co-CEO. “Sanofi has led the way in garnering insights from real-world data in R&D and we’re excited to advance our work together into its next decade."
Sanofi and Evidation announced a prior expansion of their work together in 2017, in addition to Sanofi’s investment in Evidation in the same year.
“Real-world evidence is critical to help us better understand the patient’s health and wellness journey outside of traditional healthcare visits,” said Arnaud Robert, Executive Vice President, Chief Digital Officer, Sanofi. “Through our expanded collaboration with Evidation, we can further our ambition to transform the practice of medicine by connecting more closely with patients and citizens, expanding our geographic capabilities, and increasing diversity to better represent the global population.”
This announcement comes as biopharmaceutical companies, regulators, and payers are working to develop new guidelines on how real-world data should be incorporated into the development and approval of therapeutics. Evidation and Sanofi will continue to contribute to this conversation through similar industry-leading research.
The Evidation network is made up of more than 4.4 million individuals across all 50 states, representing 9 out of every 10 U.S. ZIP codes, allowing organizations like Sanofi access to a highly engaged, diverse population and privacy-conscious research platform.
ABOUT EVIDATION
Evidation measures health in everyday life and enables anyone to participate in ground-breaking research and health programs. Built upon a foundation of user privacy and control over permissioned health data, Evidation’s platform is trusted by millions of individuals—generating data with unprecedented speed, scale, and rigor. We partner with leading healthcare companies to understand health and disease outside the clinic walls. Guided by our mission to enable and empower everyone to participate in better health outcomes, Evidation is working to bring people individualized, proactive, and accessible healthcare—faster. Founded in 2012, Evidation is headquartered in California with additional offices around the globe. To learn more, visit evidation.com, or follow us on Twitter @evidation.
Contacts
MEDIA CONTACT
Matt Miller
press@evidation.com


Partnership to create new treatment delivery options for people facing serious diseases
BOSTON, MA—February 10, 2021—i2O Therapeutics, developers of a platform for oral delivery of traditionally injectable biological drugs, announced today a research collaboration with Sanofi to investigate the oral delivery of Sanofi's Nanobody®-based medicines, which are currently administered through intravenous or subcutaneous injections.
Nanobodies - proprietary therapeutic proteins based on camelid derived immunoglobulin single variable domains - have potential uses in the treatment of a range of serious and life-threatening diseases and are being developed in many therapeutic areas including inflammation, hematology, immuno-oncology, oncology and rare diseases. The research collaboration between i2O Therapeutics and Sanofi will explore a new oral route of administering nanobodies.
“Our mission at i2O Therapeutics is to develop safe and effective oral formulations of therapies traditionally limited to injections and we are excited to partner with Sanofi to advance this mission,” said Ravi Srinivasan, co-founder and director of i2O Therapeutics.
“i2O’s ionic liquid platform opens new opportunities to orally deliver biologics, and nanobodies represent an exciting application of this platform,” said Samir Mitragotri, co-founder of i2O Therapeutics.
i2O Therapeutics announced seed funding in April 2020, which was led by Sanofi Ventures, the corporate venture capital arm of Sanofi, and JDRF T1D Fund. The company also announced a strategic investment from Colorcon Ventures, the corporate venture capital fund of Colorcon, Inc. in December 2020.
About i2O Therapeutics
i2O Therapeutics is a biotechnology company developing safe and effective oral formulations of therapies traditionally limited to injections. Using an innovative ionic liquid technology, this platform leverages the benefits of protecting the drug cargo while also transiently enhancing permeation across the epithelial lining when administered orally. i2O is focused on creating the next generation of oral peptide and protein-based therapies. Visit us at www.i2OBio.com.
About Sanofi
Sanofi is dedicated to supporting people through their health challenges. We are a global biopharmaceutical company focused on human health. We prevent illness with vaccines, provide innovative treatments to fight pain and ease suffering. We stand by the few who suffer from rare diseases and the millions with long-term chronic conditions. With more than 100,000 people in 100 countries, Sanofi is transforming scientific innovation into healthcare solutions around the globe.
Contact
Lauren Arnold
MacDougall
larnold@macbiocom.com
781-235-3060



- Kymera to receive $150 million upfront with more than $2 billion in potential milestones plus royalty payments
- Kymera to retain option during clinical development to participate equally in US cost and profit sharing
CAMBRIDGE, Mass. (July 9, 2020) – Kymera Therapeutics Inc. today announced the company has entered into a multi-program strategic collaboration with Sanofi (NASDQ: SNY) to develop and commercialize first-in-class protein degrader therapies targeting IRAK4 in patients with immune-inflammatory diseases. The companies will also partner on a second earlier stage program. Kymera will receive $150 million in cash upfront and may receive more than $2 billion in potential development, regulatory and sales milestones, as well as significant royalty payments. Kymera retains the option to participate in US development and commercialization for both programs. This includes the ability to participate equally in the costs, profits and losses after opt-in, and to co-promote partnered products in the US.
“This is an important collaboration for both companies and for the field of targeted protein degradation,” said Nello Mainolfi, Ph.D., co-founder, President and CEO of Kymera Therapeutics. “Kymera is becoming a fully integrated biotechnology company advancing a pipeline of novel therapies with the potential to transform treatment paradigms. We are excited to partner with Sanofi, an organization with world-class drug development and commercialization capabilities, to ensure maximal patient impact from two of our programs across multiple disease indications, while enabling Kymera to invest in key strategic areas to realize the broad potential of protein degrader therapies.”
Under terms of the collaboration, Sanofi will make an upfront payment of $150 million in cash to Kymera for global rights to develop its small molecule IRAK4 protein degraders in inflammation and immunology indications, and a second earlier stage undisclosed program. IRAK4 is believed to play a key role in multiple immune-inflammatory diseases, including hidradenitis suppurativa, atopic dermatitis and rheumatoid arthritis. Kymera will advance the IRAK4 program through Phase 1 clinical trials; Sanofi will assume clinical development and commercialization responsibilities thereafter. Sanofi will lead all clinical development activities for the second program. Kymera will have the option to participate in the development of both programs in the US during clinical development. Kymera will retain global rights to its IRAK4 program in oncology indications.
IRAK4 is a key protein involved in inflammation mediated by the activation of toll-like receptors (TLRs) and IL-1 receptors (IL-1Rs). While TLR and IL-1R signaling via IRAK4 is involved in the normal immune response, aberrant activation of these pathways is the underlying cause of multiple immune-inflammatory conditions. In pre-clinical studies, Kymera has shown oral daily administration of an IRAK4 degrader can lead to complete knockdown of IRAK4 in skin and immune cells in higher species and is well tolerated. Data presented at the most recent annual meetings of the American College of Rheumatology and the European Hidradenitis Suppurativa Foundation showed potent anti- inflammatory activity in both in vitro and in vivo preclinical models."
“Targeted protein degrada on is an exci ng modality. Kymera has developed an incredible drug discovery engine producing protein degraders with compelling and dieren ated pharmacology against targets that, to date, have not been op mally addressed with other therapeu c modali es,” said John Reed, Global Head of Research & Development at Sano. “We are excited to partner with the Kymera team to advance a new genera on of rst-in-class therapies with the poten al to eliminate underlying drivers of disease.”
Aquilo Partners, L.P. acted as Financial advisor to Kymera on this transac on.
# # #
About Kymera Therapeutics
Kymera Therapeutics is a biotechnology company pioneering a transformative new approach to treating previously untreatable diseases. The company is advancing the field of targeted protein degradation, accessing the body’s innate protein recycling machinery to degrade dysregulated, disease-causing proteins. Kymera’s Pegasus targeted protein degradation platform harnesses the body’s natural protein recycling machinery to degrade disease-causing proteins, with a focus on un-drugged nodes in validated pathways currently inaccessible with conventional therapeutics.
Kymera is accelerating drug discovery with an unmatched ability to target and degrade the most intractable of proteins, and advance new treatment options for patients. For more information, visit www.kymeratx.com.
About Sanofi
Sanofi is dedicated to supporting people through their health challenges. We are a global pharmaceutical company focused on human health. We prevent illness with vaccines, provide innovative treatments to fight pain and ease suffering. We stand by the few who suffer from rare diseases and the millions with long-term chronic conditions. With more than 100,000 people in 100 countries, Sanofi is transforming scientific innovation into healthcare solutions around the globe. Sanofi, empowering life. For more information, visit www.sanofi.com.


PARIS and NEW YORK – November 20, 2019 - Sanofi announced today an enterprise- wide collaboration with health care technology company Aetion that will integrate Sanofi’s real-world data platform, DARWIN, with the Aetion Evidence Platform® with the objective of advancing more efficient use of real-world evidence (RWE), facilitating regulatory-grade studies with deep transparency, and unlocking access to new real-world data.
Both companies have invested in RWE platforms, recognizing the pressing need for accurate, fast, and cost-effective research and the important role RWE could play in meeting this need. Sanofi’s DARWIN compiles and analyzes de-identified data from hundreds of millions of patients across disease states, while Aetion’s platform analyzes real-world data to produce transparent, rapid, and scientifically validated answers about the effectiveness, safety, and value of drugs. By combining these platforms, Sanofi is seeking to elevate its capabilities in conducting regulatory-grade analytics, opening new doors for the development and application of medical treatments.
“Today marks another important step in Sanofi’s digital transformation,” said Bernard Hamelin, MD, MSc, MBA, Global Head of Medical Evidence Generation, Sanofi. “By integrating these platforms, we strive to make faster, more informed decisions with the potential to lead to first-in-class and best-in-class treatments that could change the practice of medicine.”
Real-world evidence offers a view of clinical practice outside of the experimental setting, providing an opportunity to inform clinical trial development and supplement trial data with evidence of actual product use in the health care system.
“Our work with Sanofi further validates the value and potential for real-world evidence in drug development,” said Carolyn Magill, Chief Executive Officer of Aetion. “Our companies share a common goal of using the best available data to get the right treatment to the right patient as quickly and efficiently as possible.”
This collaboration between Sanofi and Aetion demonstrates leadership during a critical time. Real-world evidence is expected to play a key role in transforming the health care ecosystem, with the U.S. Food and Drug Administration (FDA) recently prioritizing efforts to incorporate RWE as a companion to clinical trial data to aid in regulatory decision making. The FDA will release its draft RWE guidance before the end of 2020.
About Aetion
Aetion is a health care technology company that delivers real-world evidence for life sciences companies, payers, at-risk providers, and regulatory agencies. The Aetion Evidence Platform analyzes data from the real world to produce transparent, rapid, and scientifically validated answers on treatments, costs, and outcomes. Founded by Harvard Medical School faculty with decades of experience in epidemiology and health outcomes research, Aetion informs health care's most critical decisions — what works best, for whom, and when — to guide treatment development, commercialization, and payment innovation into health care's modern era. Aetion is based in New York City, and backed by investors including New Enterprise Associates (NEA), Flare Capital Partners, Lakestar, Town Hall Ventures, McKesson Ventures, Sanofi Ventures, Amgen Ventures, UCB, and Horizon Health Services, Inc. Learn more at aetion.com, and follow us at @aetioninc.
About Sanofi
Sanofi is dedicated to supporting people through their health challenges. We are a global biopharmaceutical company focused on human health. We prevent illness with vaccines, provide innovative treatments to fight pain and ease suffering. We stand by the few who suffer from rare diseases and the millions with long-term chronic conditions.
With more than 100,000 people in 100 countries, Sanofi is transforming scientific innovation into healthcare solutions around the globe.
Sanofi, Empowering Life
Sanofi Media Relations Contact
Anna Robinson
Tel.: +33 (0)1 53 77 46 46
mr@sanofi.com
Aetion Media Relations Contact
202.792.7200
press@aetion.com
Sanofi Investor Relations Contact
George Grofik
Tel.: +33 (0)1 53 77 45 45
ir@sanofi.com
Sanofi Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These statements include projections and estimates and their underlying assumptions, statements regarding plans, objectives, intentions and expectations with respect to future financial results, events, operations, services, product development and potential, and statements regarding future performance. Forward-looking statements are generally identified by the words “expects”, “anticipates”, “believes”, “intends”, “estimates”, “plans” and similar expressions. Although Sanofi’s management believes that the expectations reflected in such forward- looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Sanofi, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include among other things, the uncertainties inherent in research and development, future clinical data and analysis, including post marketing, decisions by regulatory authorities, such as the FDA or the EMA, regarding whether and when to approve any drug, device or biological application that may be filed for any such product candidates as well as their decisions regarding labelling and other matters that could affect the availability or commercial potential of such product candidates, the absence of guarantee that the product candidates if approved will be commercially successful, the future approval and commercial success of therapeutic alternatives, Sanofi’s ability to benefit from external growth opportunities, to complete related transactions and/or obtain regulatory clearances, risks associated with intellectual property and any related pending or future litigation and the ultimate outcome of such litigation, trends in exchange rates and prevailing interest rates, volatile economic conditions, the impact of cost containment initiatives and subsequent changes thereto, the average number of shares outstanding as well as those discussed or identified in the public filings with the SEC and the AMF made by Sanofi, including those listed under “Risk Factors” and “Cautionary Statement Regarding Forward-Looking Statements” in Sanofi’s annual report on Form 20-F for the year ended December 31, 2018. Other than as required by applicable law, Sanofi does not undertake any obligation to update or revise any
forward-looking information or statements.



Without clinical trials, new medicine may never make it from the research lab to patients in need. These carefully designed studies can provide important data that include proper dosage, benefit to patients, and potential side effects.
There is a growing challenge, however, in finding appropriate participants, especially for treatments that target highly specific conditions affecting narrower patient populations. Right now, there are more than 40,000 clinical studies recruiting patients in the U.S. alone, with some requiring thousands of participants, each of whom must meet precise criteria to join. So it’s not surprising that 80% of these important studies are delayed due to recruitment problems, according to a study by the Center for Information and Study on Clinical Research Participation (CISCRP).
Unfortunately, those delays mean it can take longer for innovative new medicines to be studied and approved, leaving patients to wait years for new treatment options. To tackle this growing problem, Sanofi is taking a digital approach to clinical trials, partnering with Science 37, a clinical research services and technology company based in California.
Leveraging mobile technology and telemedicine capabilities, this new approach will allow Sanofi to develop “site-less” or decentralized clinical trials that are more patient friendly: easier for them to access, and eliminating many of the common impediments to participation. Using digital technologies to streamline finding and retaining participants for the entire length of a study has the potential to reduce the time required for a typical trial by at least 30%, according to Science 37.

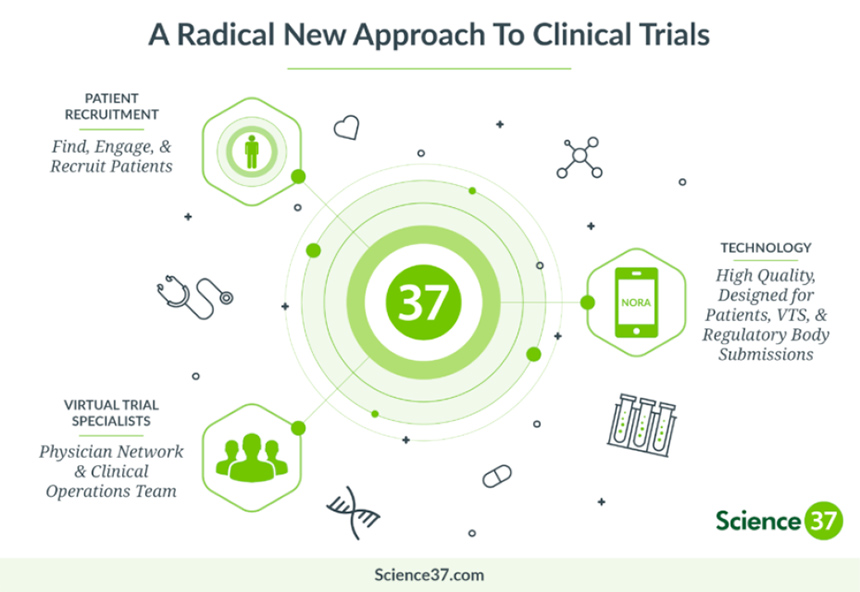
“After years invested in the lab on an innovative treatment, the clinical trials are where we finally obtain and analyze the relevant data that will let us understand how well a new treatment will benefit patients,” said Lionel Bascles, Global Head of Clinical Sciences and Operations of Sanofi. “With digital clinical trials we can get and analyze the data on how a new medicine works in the real world a lot sooner, which means patients get the medicines they need sooner.”
Going digital also eliminates a number of other hurdles to patient participation, including the most significant: geography.
Most people are eager to participate in relevant trials – 87% of patients want to do so, the CISCRP study found. Yet, 70% of potential participants live more than two hours away from the nearest study center. Because most clinical trials require patients to travel to those centers for regular tests and observations, sometimes several times each week for the duration of the trial, this distance is another challenge to patient access.
Science 37’s approach allows patients to be monitored and report to researchers via an Apple iPhone equipped with the company’s NORA® technology. Qualified study participants are provided with the phone, a data plan and any other sensors or connected devices needed for the trial, along with the medicines being researched. Participants can reach study staff at any time via the mobile device, while also remaining under the care of their local health care professionals. Mobile nurses are also sent to the participant’s home to provide services like blood draws when needed, and nearby hospitals or clinics are engaged for scans or other tests that require specialized equipment.
The patient’s data are sent securely to researchers who can immediately access information that would otherwise have to be collected by medical personnel through face-to-face interactions at study centers. This platform can also remind patients to take their study medications at the proper time, and let researchers know if participants are adhering to the study requirements.
“Our decentralized clinical trial model addresses critical shortcomings of traditional clinical trials, such as enrolling and retaining appropriate patients. Whether you live near a major research institution, or in a remote area, we make participation possible,” said Noah Craft, CEO of Science 37. “By utilizing a patient’s home in lieu of a physical trial site, we remove the burden of travel for those too sick or remote and provide access to qualified individuals who want to volunteer for a study but cannot because of geographic limitations.”
The Science 37 platform will also help engage patients who would normally not participate in clinical trials, “so our data will much more closely track the diversity of the population,” Bascles said. “In addition to reducing the burden for patients, decentralized clinical trials are far more likely to keep patients engaged for the full length of the trial, increasing the relevance and the acceptability of the data by regulators.”
Sanofi’s agreement with Science 37 covers use of its Metasite™ model and NORA technology across the U.S. with plans to expand internationally in the future. By eliminating months of searching for patients and long travel time to study sites, virtual clinical trials could reduce total trial time by as much as two years.
Partnering with Science 37 is the most recent strengthening of the relationship with Sanofi, which began last October when Sanofi’s venture capital fund, Sanofi-Genzyme BioVentures, made a minority investment in Science 37.
“Science 37 has a great track record, and they are smart and forward-thinking about developing the science around clinical trials that leverage digital technologies,” said Heather Bell, Global Head of Digital and Analytics for Sanofi. “As part of the scope of our digital strategy, we have expanded the scope of the venture fund to include digital investments, and Science 37 was our first investment since that change and we’re very excited about it.”



SEATTLE and SOUTH SAN FRANCISCO – October 16, 2014 Immune Design Corp. (NASDAQ: IMDZ), a clinical-stage immunotherapy company, today announced that it has entered into a broad collaboration for the development of a herpes simplex virus (HSV) immune therapy with Sanofi Pasteur, the vaccines division of Sanofi (EURONEXT: SAN and NYSE: SNY).
Sanofi Pasteur and Immune Design will each contribute product candidates to the collaboration: Sanofi Pasteur will contribute HSV-529, a clinical-stage replication-defective HSV vaccine product candidate, and Immune Design will contribute G103, its preclinical trivalent vaccine product candidate. The collaboration will explore the potential of various combinations of agents, including leveraging Immune Design’s GLAASTM platform, with the goal to select the best potential immune therapy for patients.
The two companies will develop the products jointly through Phase 2 clinical trials, at which point Sanofi Pasteur intends to continue development of the most promising candidate and be responsible for commercialization. Sanofi Pasteur will bear the costs of all preclinical and clinical development, with Immune Design providing a specific formulation of GLA from the GLAAS platform at its cost through Phase 2 studies. Immune Design will be eligible to receive future milestone and royalty payments on any product developed from the collaboration; other financial terms of the agreement have not been disclosed.
“Instead of being limited to a single approach, the companies are joining forces and combining multiple cutting-edge technologies with the goal to develop the most effective and safe immunotherapy to address HSV infection, a significant unmet medical need,” said Carlos Paya, M.D., Ph.D., President and Chief Executive Officer of Immune Design. “With other clinical and preclinical GLAAS-based product candidates in development, both with partners and internally at Immune Design, I believe this new collaboration continues to demonstrate the productivity and broad applicability of this platform.”
About G103 and GLAAS
G103 is a trivalent vaccine candidate consisting of recombinantly-expressed viral proteins adjuvanted with a specific formulation from Immune Design’s GLAAS platform. The combination of a novel molecular toll-like receptor 4 (TLR4) agonist with rationally selected antigens is designed to boost pre-existing T cells and trigger a broad antibody response, allowing for prophylactic and therapeutic immunization.
The GLAAS platform works in vivo and is based on a small synthetic molecule called GLA, which stands for glucopyranosyl lipid adjuvant. GLA selectively binds to the TLR4 receptor and causes potent activation of dendritic cells (DCs) leading to the production of cytokines and chemokines that drive a Th1-type immune response. When GLA is accompanied by an antigen and injected into a patient, the combination is taken up by DCs and leads to the production and expansion of immune cells called CD4 T helper lymphocytes with a Th1 phenotype. These CD4 T cells play a key role in boosting pre-existing cytotoxic T cells that are specific to the same antigen; and providing help to other immune cells, including B lymphocytes that are the precursor to antibodies, and natural killer cells that are also important in the overall immune response. Immune Design believes that GLAAS- based product candidates have the potential to target multiple types of cancer, as well as infectious, allergic and autoimmune diseases. Product candidates leveraging GLAAS’ core technology have now
been evaluated in over 1000 subjects in Phase 1 and Phase 2 trials.
About Immune Design
Immune Design (NASDAQ: IMDZ) is a clinical-stage immunotherapy company employing next- generation in vivo approaches to enable the body’s immune system to fight disease. The company’s technologies are engineered to activate the immune system’s natural ability to create and/or expand antigen-specific cytotoxic T cells, while enhancing other immune effectors, to fight cancer and other chronic diseases. Immune Design’s three on-going immuno-oncology clinical programs are the product of its two synergistic discovery platforms: ZVexTMand GLAASTM, the fundamental technologies of which were licensed from the California Institute of Technology and the Infectious Disease Research Institute (IDRI), respectively. Immune Design has offices in Seattle and South San Francisco. For more information, visit www.immunedesign.com.
Immune Design Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as "may," "will," "expect," "plan," "anticipate," "estimate," "intend", “believe” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. These forward-looking statements are based on Immune Design’s expectations and assumptions as of the date of this press release. Each of these forward-looking statements involves risks and uncertainties. Actual results may differ materially from these forward-looking statements. Forward-looking statements contained in this press release include statements regarding efforts to develop products under the collaboration, the potential receipt of milestone and royalty payments and the potential to develop new therapeutics. Factors that may cause actual results to differ from those expressed or implied in the forward-looking statements in this press release are discussed in Immune Design’s filings with the U.S. Securities and Exchange Commission (the “SEC”), including the "Risk Factors" section of Immune Design’s Quarterly Report on Form 10-Q filed with the SEC on September 8, 2014 and in any subsequent filings with the SEC. Except as required by law, Immune Design assumes no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available.
# # #
For Immune Design:
Media Contact Julie Rathbun
Rathbun Communications
julie@rathbuncomm.com
206-769-9219
Investor Contact Robert H. Uhl
Westwicke Partners
robert.uhl@westwicke.com
858-356-5932



CAMBRIDGE, Mass. and SEATTLE and SOUTH SAN FRANCISCO, Calif., Aug. 7, 2014 (GLOBE NEWSWIRE) -- Sanofi
(EURONEXT:SAN) (NYSE:SNY) and Immune Design (Nasdaq:IMDZ), a clinical-stage immunotherapy company, today announced that they have entered into a licensing agreement for use of Immune Design's GLAASTM discovery platform to develop therapeutic agents to treat a selected food allergy.
The incidence of food allergies is increasing worldwide in both developed and undeveloped countries, and especially in children.1 Globally, experts believe 220-250 million people may suffer from food allergies.2,3 In the United States alone, as many as 15 million people have food allergies,4 with allergic reactions resulting in an emergency room visit every three minutes and averaging more than 200,000 emergency room visits per year.5
"This is an exciting time in the area of immunology research, and our relationship with Immune Design is a great example of how Sanofi has changed our approach to R&D," said Kurt Stoeckli, vice president and head of Global Bio Therapeutics Organization, Sanofi. "With this partnership, we are able to tap into breakthrough science that holds great potential to transform how food allergies are treated, and the lives of those people affected. This kind of innovation is central to our new approach."
Under terms of the agreement, Immune Design has granted Sanofi an exclusive license to discover, develop and commercialize products to treat a selected food allergy. The company has received an undisclosed upfront payment and will be eligible to receive development and commercialization milestones totaling US $168 million, as well as tiered royalties on sales of approved products.
"Our fourth agreement for the use of the GLAAS platform further demonstrates the broad applicability of this approach not only in cancer and infectious diseases, but now in allergic diseases as well," said Stephen Brady, chief business officer at Immune Design. "Due to the immune dysfunction leading to allergic diseases, GLAAS' mechanism of action is well suited to correct the imbalance, allowing for the potential of new therapeutics in the targeted indication that currently uses century-old technologies. We are pleased that Sanofi has decided to develop products for this often life-threatening and growing food allergy."
Under an existing collaborative research arrangement, Sanofi and Immune Design have generated a large set of preclinical data demonstrating that certain formulations within GLAAS, when given prophylactically or therapeutically, can shift the immune responses in a way that may result in significant protection and reduction from allergy symptoms.
About Sanofi
Sanofi, an integrated global healthcare leader, discovers, develops and distributes therapeutic solutions focused on patients' needs. Sanofi has core strengths in the field of healthcare with seven growth platforms: diabetes solutions, human vaccines, innovative drugs, and consumer healthcare, emerging markets, animal health and the new Genzyme. Sanofi is listed in Paris (EURONEXT:SAN) and in New York (NYSE:SNY).
About GLAAS
Immune Design's GLAAS platform works in vivo and is based on a small synthetic molecule called GLA, which stands for glucopyranosyl lipid adjuvant. GLA selectively binds to the TLR4 receptor and causes potent activation of dendritic cells (DCs) leading to the production of cytokines and chemokines that drive a Th1-type immune response. When GLA is accompanied by an antigen and injected into a patient, the combination is taken up by DCs and leads to the production and expansion of immune cells called CD4 T helper lymphocytes with a Th1 phenotype. These CD4 T cells play a key role in boosting pre- existing CTLs that are specific to the same antigen; and providing help to other immune cells, including B lymphocytes that are the precursor to antibodies, and natural killer cells that are also important in the overall immune response. Immune Design believes that GLAAS product candidates have the potential to target multiple types of cancer, as well as infectious, allergic and autoimmune diseases. GLAAS-based product candidates have now been evaluated in over 1000 subjects in Phase 1 and Phase 2 trials demonstrating an acceptable safety profile and efficacy.
About Immune Design
Immune Design (Nasdaq:IMDZ) is a clinical-stage immunotherapy company employing next-generation in vivo approaches to enable the body's immune system to fight disease. The company's technologies are engineered to activate the immune system's natural ability to create tumor-specific cytotoxic T cells, while enhancing other immune effectors, to fight cancer and other chronic diseases. Immune Design's three on-going Immuno-oncology clinical programs are the product of its two synergistic discovery platforms: DCVexTMand GLAASTM, the fundamental technologies of which were licensed from the California Institute of Technology and the Infectious Disease Research Institute, respectively. Immune Design has offices in Seattle, Washington and South San Francisco, California. For more information, visit www.immunedesign.com.
Sanofi Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These statements include projections and estimates and their underlying assumptions, statements regarding plans, objectives, intentions and expectations with respect to future financial results, events, operations, services, product development and potential, and statements regarding future performance. Forward-looking statements are generally identified by the words "expects", "anticipates", "believes", "intends", "estimates", "plans" and similar expressions. Although Sanofi's management believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Sanofi, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include among other things, the uncertainties inherent in research and development, future clinical data and analysis, including post marketing, decisions by regulatory authorities, such as the FDA or the EMA, regarding whether and when to approve any drug, device or biological application that may be filed for any such product candidates as well as their decisions regarding labelling and other matters that could affect the availability or commercial potential of such product candidates, the absence of guarantee that the product candidates if approved will be commercially successful, the future approval and commercial success of therapeutic alternatives, the Group's ability to benefit from external growth opportunities, trends in exchange rates and prevailing interest rates, the impact of cost containment policies and subsequent changes thereto, the average number of shares outstanding as well as those discussed or identified in the public filings with the SEC and the AMF made by Sanofi, including those listed under "Risk Factors" and "Cautionary Statement Regarding Forward-Looking Statements" in Sanofi's annual report on Form 20-F for the year ended December 31, 2013. Other than as required by applicable law, Sanofi does not undertake any obligation to update or revise any forward-looking information or statements.
Immune Design Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as "may," "will," "expect," "plan," "anticipate," "estimate," "intend", "believe" and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. These forward-looking statements are based on Immune Design's expectations and assumptions as of the date of this press release. Each of these forward-looking statements involves risks and uncertainties. Actual results may differ materially from these forward-looking statements. Forward-looking statements contained in this press release include statements regarding the receipt of milestone and royalty payments, the potential to develop new therapeutics and the potential of any future products to prevent and reduce allergy symptoms. Factors that may cause actual results to differ from those expressed or implied in the forward-looking statements in this press release are discussed in Immune Design's filings with the U.S. Securities and Exchange Commission, including the "Risk Factors" contained therein. Except as required by law, Immune Design assumes no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available.
References
- "Food Allergy - A Rising Global Health Problem," World Allergy Week 2013. 8-14 April 2013. http://www.worldallergy.org/UserFiles/file/WorldAllergyWeek2013final.pdf. Accessed online, July 28, 2014.
- Mills EN, Mackie AR, Burny P, Beyer K, Frewer L et al. "The prevalence, cost and basis of food allergy across Europe." Allergy 2007; 62:717-722.
- Fiocchi A, Sampson HA. "Food Allergy", Section 2.5, in WAO White Book on Allergy, Pawankar R, Canonica GW, Holgate ST, and Lockey RF, editors (Milwaukee, Wisconsin: World Allergy Organization, 2011), pp. 47-53.
- National Institute of Allergy and Infectious Diseases, National Institutes of Health. Report of the NIH Expert Panel on Food Allergy Research. 2006. Accessed online, July 25, 2014. http://www.niaid.nih.gov/topics/foodallergy/research/pages/reportfoodallergy.aspx
- 5. Clark S, Espinola J, Rudders SA, Banerji, A, Camargo CA. Frequency of US emergency department visits for food-
related acute allergic reactions. J Allergy ClinImmunol. 2011; 127(3):682-683.
CONTACT:
Amy BA, Ph.D.
Sanofi Global R&D Communications
Amy.Ba@sanofi.com
Tel: 646-207-4935
Julie Rathbun
Rathbun Communications (Immune Design)
julie@rathbuncomm.com
Tel: 206-769-9219


















